You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
BREAKING: FDA AUTHORIZED COVID-19 BIVALENT VAX FOR BABIES
- Thread starter Goldhedge
- Start date
Welcome to the Precious Metals Bug Forums
Welcome to the PMBug forums - a watering hole for folks interested in gold, silver, precious metals, sound money, investing, market and economic news, central bank monetary policies, politics and more.
Why not register an account and join the discussions? When you register an account and log in, you may enjoy additional benefits including no Google ads, market data/charts, access to trade/barter with the community and much more. Registering an account is free - you have nothing to lose!
Oldmansmith
Fly on the Wall
- Messages
- 50
- Reaction score
- 170
- Points
- 103
Makes perfect sense. Give an untested jab to an age group that has no chance of dying from the disease that the jab won’t stop you from getting. I should have invested in Phi$er.
This was announced Thursday, December 8:

 www.fda.gov
www.fda.gov
...
The monovalent Moderna COVID-19 Vaccine is authorized as a two-dose primary series in individuals six months of age and older and as a third primary series dose for individuals 6 months of age and older who have been determined to have certain kinds of immunocompromise. With today’s authorization, the Moderna COVID-19 Vaccine, Bivalent is now authorized for administration in individuals 6 months through 5 years of age as a single booster dose at least 2 months after completion of primary vaccination with the monovalent Moderna COVID-19 Vaccine. The Moderna COVID-19 Vaccine, Bivalent is also authorized for use in individuals 6 years and older as a single booster dose at least two months after completion of either primary vaccination with any authorized or approved COVID-19 vaccine, or receipt of the most recent booster dose with any authorized or approved monovalent COVID-19 vaccine.
For the authorization of a single booster dose of the Moderna COVID-19 Vaccine, Bivalent for children 6 months through 5 years of age, the FDA relied on immune response data that it had previously evaluated from a clinical study in adults of a booster dose of Moderna’s investigational bivalent COVID-19 vaccine that contained a component corresponding to the original strain of SARS-CoV-2 and a component corresponding to the omicron lineage BA.1.
In addition, the FDA conducted an analysis of data from a clinical study that compared the immune response among 56 study participants 17 months through 5 years of age who received a single booster dose of monovalent Moderna COVID-19 Vaccine at least six months after completion of a two-dose primary series of the vaccine to the immune response among approximately 300 study participants 18 through 25 years of age who had received a two-dose primary series of monovalent Moderna COVID-19 Vaccine in a previous study which determined the vaccine to be effective in preventing COVID-19. The immune response to the booster dose of monovalent Moderna COVID-19 Vaccine in the 17 months through 5 years age group was comparable to the immune response to the two-dose primary series in the adult participants.
The safety of a single booster dose of the Moderna COVID-19 Vaccine, Bivalent for children 6 months through 5 years of age is supported by safety data from a clinical study which evaluated a booster dose of Moderna’s investigational bivalent COVID-19 vaccine (original and omicron BA.1), safety data from clinical trials which evaluated primary and booster vaccination with the monovalent Moderna COVID-19 Vaccine, and postmarketing safety data with the monovalent Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent.
In one clinical study, the safety of a single booster dose of monovalent Moderna COVID-19 Vaccine was evaluated in 145 clinical study participants 6 months through 5 years of age who received a booster dose of monovalent Moderna COVID-19 Vaccine at least six months after completion of the monovalent Moderna COVID-19 Vaccine two-dose primary series. The most commonly reported side effects after a booster dose of the monovalent Moderna COVID-19 Vaccine across this age group included pain, redness and swelling at the injection site, swelling/tenderness of the lymph nodes of the injected arm or thigh, and fever. In clinical study participants 17 months through 36 months of age, other commonly reported side effects included irritability/crying, sleepiness, and loss of appetite. In clinical trial participants 37 months through 5 years of age, other commonly reported side effects included fatigue, headache, muscle pain, joint pain, chills, and nausea/vomiting.
The data accrued with the investigational Moderna bivalent COVID-19 vaccine (original and omicron BA.1) and with the monovalent Moderna COVID-19 Vaccine are relevant to the Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
...

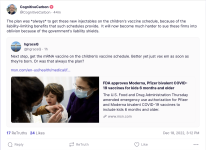
Coronavirus (COVID-19) Update: FDA Authorizes Updated (Bivalent) COVID-19 Vaccines for Children Down to 6 Months of Age
The FDA amended the EUAs of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age.
Oldmansmith
Fly on the Wall
- Messages
- 50
- Reaction score
- 170
- Points
- 103
Yes, but it bears repeating.This was announced Thursday, December 8:

Coronavirus (COVID-19) Update: FDA Authorizes Updated (Bivalent) COVID-19 Vaccines for Children Down to 6 Months of Age
The FDA amended the EUAs of the updated (bivalent) Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children down to 6 months of age.www.fda.gov
Not even hiding it now.... The Fraud and Death Admin is going full Kill Bill now.
- Messages
- 18,141
- Reaction score
- 10,928
- Points
- 288